Abstract
Background:
B-cell maturation antigen (BCMA) directed chimeric antigen receptor T-cell therapy (CAR-T) has shown unprecedented efficacy in multiple myeloma (MM). CAR-T toxicities in the acute period, cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and macrophage activation syndrome (MAS) are managed by tocilizumab, steroids, and/or anakinra. However, since steroids have been shown to inhibit T cell activity (Ashwell et al. 2000), their use to mitigate these toxicities may have deleterious effects on CAR-T efficacy. Previous studies which examined steroid effects on CAR-T therapy in other hematologic malignancies have shown mixed results (Liu et al. 2020, Strati et al. 2021, Topp et al. 2019). We performed a single-center retrospective analysis of steroid use in MM patients (pts) during BCMA CAR-T hospitalization.
Methods:
All patients treated at UCSF with a BCMA CAR-T for MM from 11/1/2017-3/31/2021 were analyzed for steroid use during CAR-T hospitalization and treatment outcomes. Per institutional policy, all CAR-T pts were hospitalized for at least 14 days post-CAR-T infusion. Baseline pt characteristics, steroid usage, CAR-T toxicities and its treatment were collected. Overall response rate (ORR) was determined according to International Myeloma Working Group criteria. Overall survival (OS), progression-free survival (PFS) and time-to-next treatment (TTNT) were summarized using Kaplan-Meier methods and compared using log-rank tests. Wilcoxon rank-sum and Fisher's exact tests were used to compare continuous and categorical variables, respectively.
Results:
Of the 62 CAR-T pts, 24 (38.7%) received steroids during CAR-T hospitalization. Fifteen (62.5%), 5 (20.8%), 1 (4.2%) and 3 (12.5%) pts were given steroids respectively for CRS only, CRS and ICANS, CRS and MAS, or ICANS only. CAR-T toxicities included: 52 pt with CRS (27 gr 1, 25 gr 2); 8 pt with ICANS (3 gr 1, 3 gr 2, 2 gr 3) and 9 pt with MAS (3 gr 1, 2 gr 2, 3 gr 3, 1 gr 4). All pts received only dexamethasone (dex) except for 1 for whom solumedrol was converted into dex equivalent units. Median time to steroid initiation after CAR-T infusion was 2 days (0-10), median total days on steroids was 4 (1-10), and median cumulative steroid dose was 60mg (10-498).
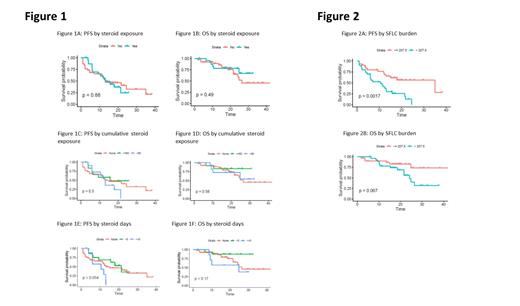
Overall, the results showed no significant difference in ORR (95.8% v 84.2, p=0.2), PFS (13.1 v 13.2 mo, p=0.9) (Fig. 1A), OS (not reached (NR) v 26.4 mo, p=0.5) (Fig. 1B) or TTNT (10.5 v 7.0, p=0.3) when pts received steroids compared to no steroids. There were also no significant differences in ORR, PFS, OS, or TTNT based on cumulative dose of steroid received. Pts given 0 v ≤ 60 v > 60mg cumulative steroid dose had ORR of 84.2%, 100%, 90.9% (p=0.4) and median PFS of 13.2, 15.7 and 13 mo (p=0.5) (Fig. 1C), respectively. Similarly, median OS (Fig. 1D) and TTNT was 26.3 mo, NR, NR (p=0.6) and 22.8, 17.5, 15.1 mo (p=0.6) for the three groups, respectively.
Pts who received steroids for 0 vs 1-5 vs ≥5 days had ORR of 84.2%, 100% and 85.7% (p=0.2). There was no statistically significant difference in median PFS (13.2, 21.4, and 10.6 mo (p=0.05)) (Fig. 1E) or median OS (26.4, NR, and 24.8 mo (p = 0.2)) (Fig. 1F). Median TTNT was statistically significant at 22.8, 24.6, and 12.5 mo (p = 0.04).
Median length of hospitalization was 14 days for both steroid-treated and non-steroid treated pts. Median disease burden (as measured by pre-CAR-T serum free light chains (SFLC)) also did not differ based on steroid treatment (195.9 mg/L vs 207.5 mg/L, p=0.9). Median follow-up time for the whole cohort was 19.0 mo (range 1.2-42.9). Forty-three (69%) pts died and 36 (58%) pts progressed through CAR-T.
Lastly rates and timing of CRS, ICANS, and MAS, as well as tocilizumab and anakinra doses, initiation dates, and duration were not significantly associated with worsened ORR, PFS or OS. Pts with a high (>207.5 mg/L) pre-CAR-T SFLC were associated with a shortened PFS (10.6 v 35 mo, p=0.002) (Fig. 2A), OS (24.2 mo v NR, p=0.07) (Fig. 2B) and TTNT (11.9 v 41.4 mo, p=0.002) compared to those with low disease burden (≤207.5 mg/L).
Conclusions:
In conclusion, our retrospective study showed that steroid use in general is not significantly associated with worsened ORR, PFS, OS, and TTNT in pts receiving BCMA targeted CAR-T for MM. There may be an impact on PFS and TTNT when the total time on steroids is ≥ 5 days. Future larger studies are needed to examine the effect of steroid exposure and duration on BCMA CAR-T efficacy.
Lo: Oncopeptides: Consultancy; EUSA Pharma: Consultancy. Martin: GlaxoSmithKline: Consultancy; Amgen: Research Funding; Janssen: Research Funding; Oncopeptides: Consultancy; Sanofi: Research Funding. Wolf: Adaptive Biotechnologies: Consultancy; Teneobio: Consultancy; Sanofi: Consultancy; Amgen: Consultancy. Chung: Caelum: Research Funding. Shah: Nektar: Research Funding; Oncopeptides: Consultancy; Poseida: Research Funding; Kite: Consultancy; Sanofi: Consultancy; Sutro Biopharma: Research Funding; Janssen: Research Funding; Karyopharm: Consultancy; Precision Biosciences: Research Funding; Indapta Therapeutics: Consultancy; GSK: Consultancy; CSL Behring: Consultancy; CareDx: Consultancy; BMS/Celgene: Research Funding; Bluebird Bio: Research Funding; Amgen: Consultancy; Teneobio: Research Funding. Wong: Amgen: Consultancy; Genentech: Research Funding; Fortis: Research Funding; Janssen: Research Funding; GloxoSmithKlein: Research Funding; Dren Biosciences: Consultancy; Caelum: Research Funding; BMS: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal